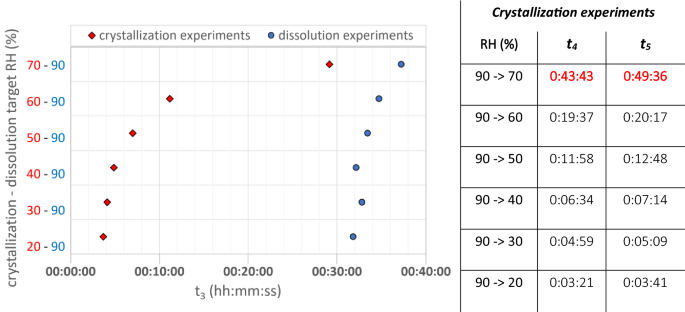
NaCl-related weathering of stone: the importance of kinetics and salt mixtures in environmental risk assessment | Heritage Science | Full Text

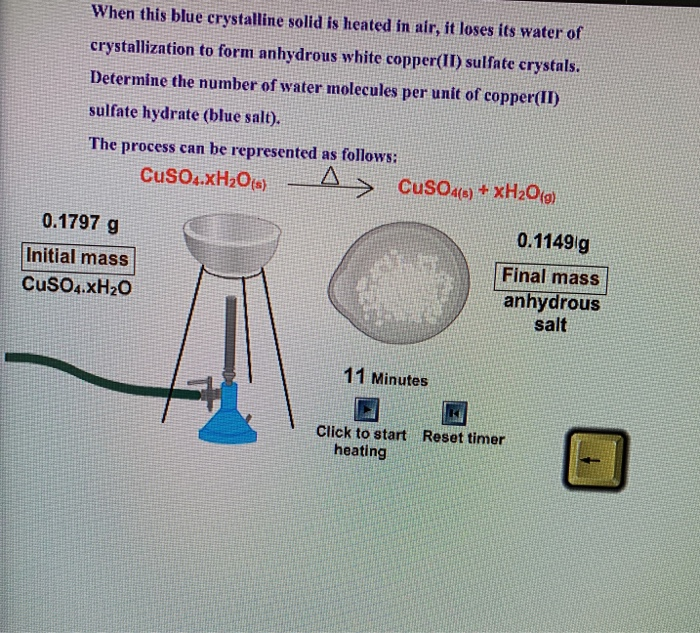
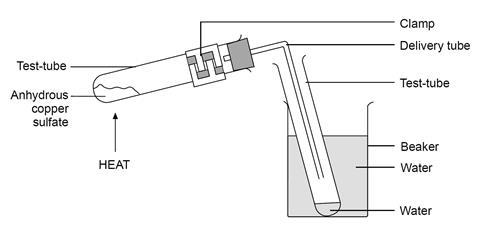
The metal salt A is in blue colour. When salt A is heated strongly over a burner then a substance B present in it is eliminated and a white powder C is
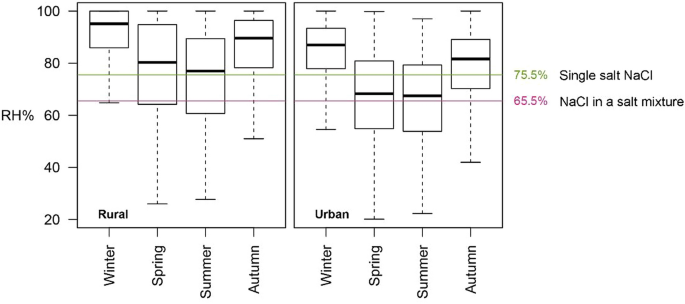
NaCl-related weathering of stone: the importance of kinetics and salt mixtures in environmental risk assessment | Heritage Science | Full Text
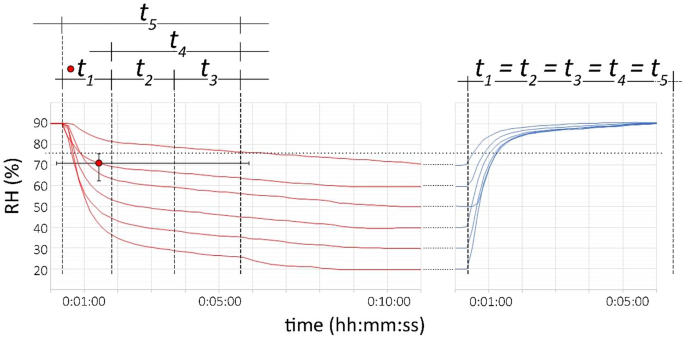
NaCl-related weathering of stone: the importance of kinetics and salt mixtures in environmental risk assessment | Heritage Science | Full Text

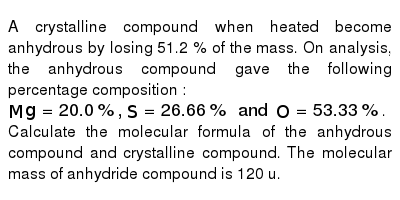
A crystalline salt when heated becomes anhydrous and losses 51.2% - CBSE Class 11 Chemistry - Learn CBSE Forum
If the crystalline salt NA2SO4.xH2O on heating loses 55.9% of its mass, then what is the formula of the salt? - Quora